
A Bordered Sacrum Foam dressing that consists of a a soft silicone wound contact layer (Safetac), which is a unique adhesive technology which minimises pain to patients and trauma to wounds and the surrounding skin at dressing removal. An absorbent polyurethane foam pad containing silver sulphate and coloured by activated carbon, a layer with super absorbent fibres, a non woven and a breathable and water proof film. Silver sulphate creates an effective bacterial barrier and inactivate a wide range of wound related pathogens (bacteria and fungi), as shown in vitro. By reducing the number of micro organisms, it may also reduce odour. Mepilex Border Sacrum Ag has been shown to inactivate wound related pathogens up to 7 days in vitro.
Designed for the management of medium to high exuding leg and foot ulcers, pressure ulcers, malignant wounds, partial thickness burns, traumatic and surgical wounds where a moist environment, exudate handling, gentle fixation and an antimicrobial action is indicated. Mepilex Border Ag may be used on infected wounds as part of a treatment regimen under supervision of a qualified health care professional. Mepilex Border Ag can be used under compression bandaging.
Hypersensitivity to dressing materials

Soft, sterile non-woven dressing made from two layers of 1.2% ionic silver-impregnated Hydrofiber; sodium carboxymethylcellulose and enhanced with More Than Silver antibiofilm technology, stitched together with cellulose-strengthening fibres. Absorbs wound fluid, and disrupts and breaks down biofilm to expose and kill bacteria, while preventing biofilm re-formation. Provides nine times stronger tensile strength and 50% greater absorption than Aquacel Ag.
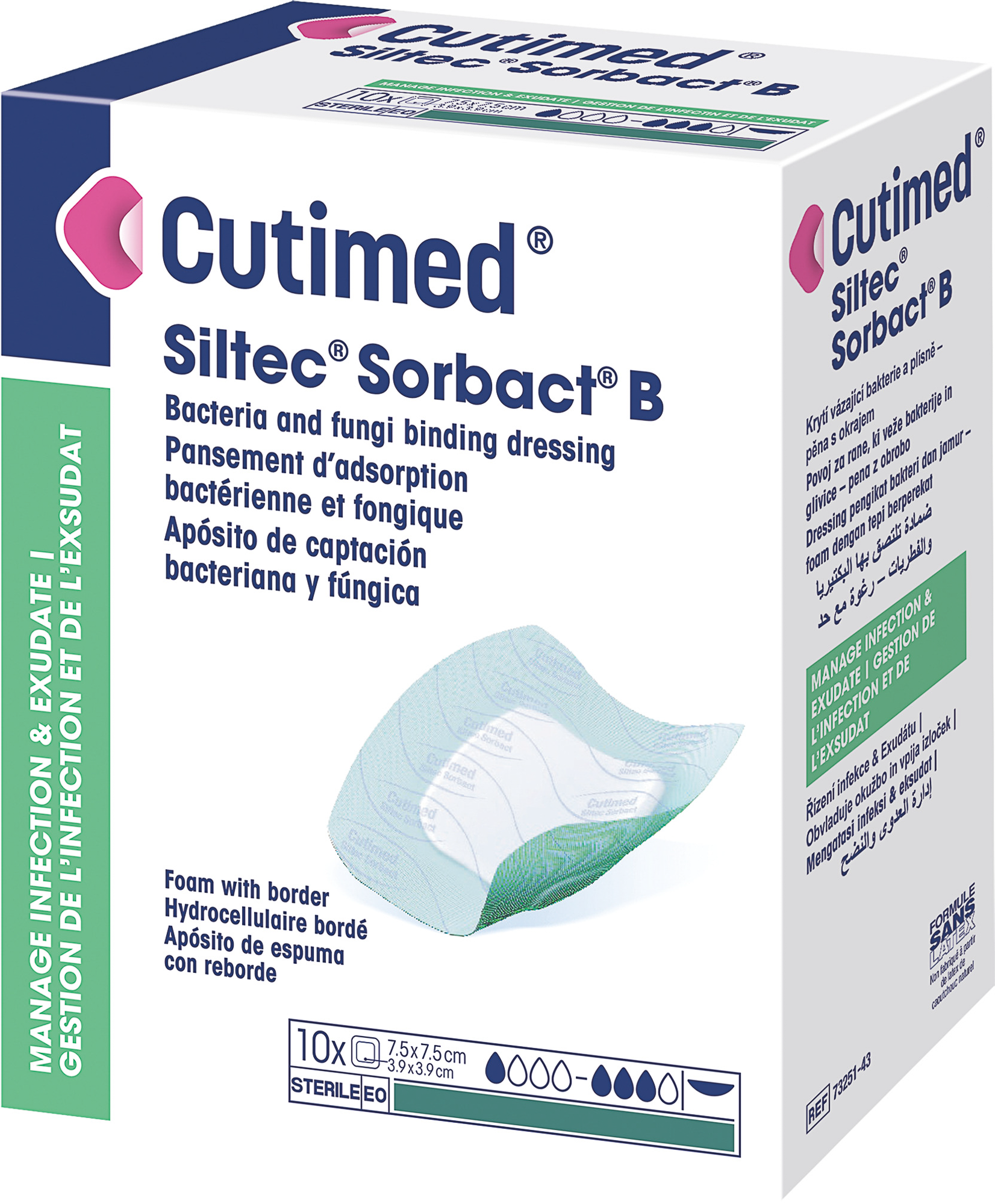
A sterile, bacteria and fungi binding wound dressing with a DACC Contact layer combined with a foam and a silicone adhesive border.



